EMVision Medical Devices (ASX: $EMV) advances towards validation trial for neuroimaging device
- Author: Stock Piper
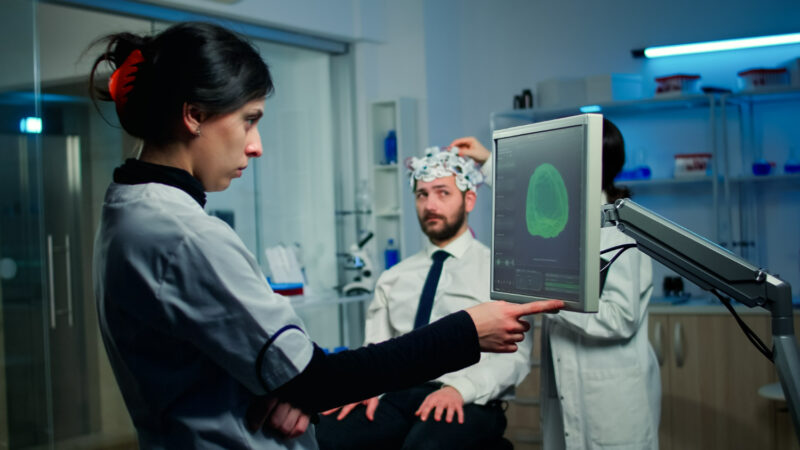
EMVision progresses with FDA-backed trial
EMVision Medical Devices (ASX:EMV) is making significant strides toward a validation trial for its portable neuroimaging device, emul TM. Following a productive consultative meeting with the U.S. Food and Drug Administration (FDA), the company is moving forward with plans to demonstrate the device’s effectiveness in detecting acute strokes. The trial is a crucial step in achieving De Novo regulatory clearance.
EMVision's strategic trial plans and vision
EMVision Medical Devices is gearing up for a validation trial of its emul TM device, following a successful engagement with the FDA. The trial will involve multiple centers and up to 300 participants, aiming to establish the device's sensitivity and specificity in stroke detection. Preparations are underway, including ethics approvals and site engagements in the U.S. The company, which operates from Sydney and Brisbane, focuses on developing portable, non-invasive neuroimaging solutions. EMVision aims to transform stroke diagnosis and treatment, with ongoing efforts to meet regulatory standards and enhance device usability. Despite potential risks, such as clinical trial uncertainties and regulatory challenges, EMVision is committed to advancing its innovative medical technology.
Executive insights on FDA engagement
The meeting with the FDA was part of their Q-submission program, designed to align our trial design and execution with FDA requirements. This engagement is key to ensuring our device meets the necessary regulatory standards,' said the company's executive team.


